



|

|
|

|
|
|
Go To | Photo Products Technical Information |
LabLine Training Index |
Part Three
|
| 1. Development | 4. Washing/Washless Stabilizer |
| 2. Bleaching | 5. Dye Stabilization (C-41 only) |
| 3. Fixing |
Some processes combine one or more of these stages with others in the process. For example, several of the minilab film processor models have combined the wash and dye stabilization into a single step or bath and in the RA-4 and EP-2 processes, the bleaching and fixing stages are combined as a single chemical bath (see below).
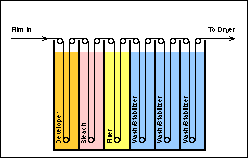
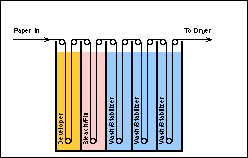
Film and paper emulsions and the film and paper processes are very complex. This complexity requires that the two processes be carefully monitored. Process Monitoring is one of the most critical procedures conducted in the operation of a photo processing lab. Product Quality is measured, evaluated and controlled through this procedure. The successful lab will place process monitoring as a first priority. Good process monitoring is the only mechanism by which a lab can produce a product of good quality with a minimum of waste and cost. Good and consistent product quality means good business. It represents a professional attitude towards the customer. It yields satisfied customers who receive good results from their exposed rolls of film which in turn guarantees new and repeat business thereby insuring the continued success of the lab.
To accomplish this, each of the individual chemical solutions (i.e. C-41 developer) must be operated and maintained within predetermined specifications. These are typically published by the manufacturer of the film, paper, chemicals or equipment. (The exact specifications utilized in any lab will depend on the individual film, paper, chemical and equipment mix for that lab.) Those specifications include among others; processing times for each step, operating temperatures for the solutions, solution agitation specifications, solution replenishment rates and chemical mixing recommendations. These are commonly referred to as Processing Factors. Process monitoring is simply a procedure by which these factors are monitored and adjusted as necessary to keep the process running within specifications.
To produce a top quality product, a sound understanding of processing factors and their effects on film or paper is essential to the lab operator. However, to fully understand how each of the processing factors affects film or paper, it is first necessary to have a basic understanding of film and paper composition and construction, and an understanding of the function of each of the individual chemical steps in the processes.
Color photographic films are made up of essentially two primary components: a transparent base and a multi-layer, gelatin-silver halide emulsion structure. The base is typically a cellulose acetate or polyester material which provides a support structure for the emulsion. The emulsion consists of several different materials or compounds that are applied to the base in individual layers during the manufacturing process of the film.
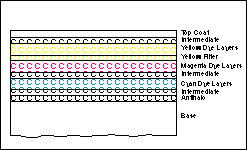
Each of the individual layers in the emulsion has a specific purpose and make-up. The most important of these layers are the light sensitive dye producing layers. Within these layers are suspended light sensitive, silver halide (silver bromide, silver chloride or silver iodide) crystals or grains and color couplers (which are transformed into colored dyes at development). There are at least three light sensitive layers, one sensitive to each of the three primary colors of light; red, green and blue. In modern films, each of these light sensitive layers is actually made of multiple layers each sensitive to varying degrees of light intensity. This improves the tonal reproduction, grain structure and sharpness of the films. In these layers, through exposure and processing, the actual dyes, cyan, magenta and yellow, are formed to make up the actual negative image.
The green and red layers, by their nature, are also sensitive to blue light. Immediately under the blue sensitive layer is a yellow filter layer. This yellow filter absorbs all the blue light so it does not reach the green and red layers. There are also several layers called intermediate layers, which primarily prevent the other layers from intermixing or reacting with each other.
Next to the base is an antihalation layer. This layer absorbs any light that passes through the emulsion and prevents it from entering into the base and then reflecting back into the emulsion which would create a halo pattern around high intensity (density) images in the film.
On the surface of the film is a top coat. It is a protective layer that prevents damage to the emulsion that could occur during camera loading, processing and printing.
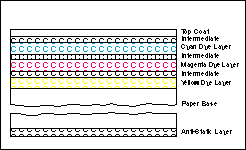
The base is, in most cases, a paper material that has a resin coating. The resin coating prevents the paper from absorbing any of the chemical solutions during processing. The base typically will have an anti-static layer coated on the back side. It prevents static electricity from building up and discharging on the surface of the paper as it travels through the printer.
Due to the complex, multi-layered emulsions of film and paper, and the sensitivity of the emulsions to chemical solution variances, each of the processing factors plays a critical role in proper development and further processing of these two light sensitive medians. Proper control of each of the processing factors for each of the chemical solutions is the key to good quality processing. Let us now look at each of the individual chemical solutions and the role they play in film and paper processing.
When the silver halide grains in a light sensitive material are exposed to light, a potential for an image is formed. This potential image is called a latent image. During processing, the color developing agent converts the exposed silver halide grains to metallic silver through a chemical
reaction called reduction. During this reaction the developing agent becomes oxidized. The oxidized developing agent then reacts with the color couplers in the emulsion to form a dye image. A cyan colored dye is formed in the red sensitive layer, a magenta dye in the green sensitive layer and a yellow dye in the blue sensitive layer.
These steps can be represented by the following:
B. Color Negative Bleach
Bleach is the second chemical step in the color negative film process. The bleach solution first stops the development process through a change in pH and the dilution of the remaining developer solution contained in the emulsion. The pH of the developer is approximately 10 (alkaline) and the pH of the bleach is approximately 5 (acidic). Developing agents must be alkaline to be active.
The bleach has as its primary function the task of converting the developed metallic silver in the film to a nonmetallic silver compound which can then be removed from the film by the fixer leaving only the dye image. (In some films, the yellow filter and antihalation layers are also silver compounds that the bleach also converts to nonmetallic silver compounds.) The compound that performs this function is referred to as the bleaching agent or oxidizing agent. During this process the metallic silver takes on a halide ion and becomes a nonmetallic compound, and the bleaching agent is reduced from an active form to an inactive form. To maintain a proper balance of active bleaching agents, air is usually bubbled through the bleach during the process. The air converts the inactive bleach to an active form.
These steps can be represented by the following:
C. Color Negative Fixer
The fixer converts the unexposed silver halides and the bleach converted silver halides in the film to complex soluble silver salts which then dissolve out of the film into the fixing solution. This silver is later removed from the fixing solutions through various silver recovery processes.
D. Color Paper Bleach-Fix
The color paper bleach-fix is essentially a combination of the film bleach and fixer into a single solution. This combined solution performs the same functions in the paper process as the separate solutions in the film process.
E. Color Film and Paper Wash and Washless Stabilizers
In the emulsions of both film and paper that have been processed, there still remains residual quantities of bleach-fixes, fixers, soluble silver complexes and other processing chemicals. These must be removed from the emulsion prior to drying to prevent image staining and fading. This is done through diffusion of water into the emulsion which removes these compounds. In the past, this was simply done with water. However, due to increased water costs and conservation efforts, new "washless" stabilizers were developed to enable photographic films and papers to be washed with reduced quantities of water. Most small photofinishing operations employ these washless stabilizers in place of water washes.
F. Color Film Stabilizer
In most of the current processing systems in smaller labs, this step is combined with the washing step in the process. However in larger labs the film passes through a final stabilizer solution following the wash prior to the film dryer. In both systems, the stabilizer compounds perform several functions. The stabilizer contains an agent that helps stabilize the dyes in the film. This prevents the dyes from fading or changing color over time. The stabilizer contains a compound which hardens the emulsion protecting it from scratches during drying and further handling. It also contains a wetting agent that allows for uniform, spot free drying. In those systems in which the wash and stabilizer are combined, the stabilizer contains an agent that prevents algae growth in the processing tank. Algae will cause a stain in the film emulsion and can attack the dyes.
Next Issue:
Processing Factors
2) Processing Factors may include:
3) Color films contain a _____________, while color papers do not.
4) Most color papers have a:
5) A color developer is also referred to as a:
6) In the film process air is bubbled through the bleach to:
7) The wash step in either the film or paper process:
8) A film stabilizer contains:
1 = b
2 = b
3 = e
4 = d
5 = a
6 = c
7 = d
8 = a
Go To | Training Guide 1 | Training Guide 2 | Training Guide 4 | Training Guide 5 | Training Guide 6 |

Copyright 1996 Imation. All rights reserved.












